rfid chip in medication So, a “universal” RFID tag for medication management would require standards. Additionally, those various frequencies have different capabilities and limits, such as reading through glass, or water. There are basically two types of RFID tags, passive and active. ACS ACR1552U USB-C NFC Reader IV . The ACR1252U USB NFC Reader III is an NFC Forum-certified PC-linked reader, developed based on 13.56Mhz contactless technology. It has a SAM (Secure Access Module) slot which can .The ACR122U NFC Reader is a PC-linked contactless smart card reader/writer developed based on 13.56 MHz Contactless (RFID) Technology. Compliant with the ISO/IEC18092 standard for Near Field Communication (NFC), it supports not only MIFARE® and ISO 14443 A and B .
0 · Thoughts on use of RFID in Medication Management
1 · FDA approves pill with sensor that digitally tracks if patients
I have a weird problem that I couldn't find any solutions online, so far. NFC function on my Samsung s20fe simply doesn't work. When I try to use it.
The U.S. Food and Drug Administration today approved the first drug in the U.S. with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible. So, a “universal” RFID tag for medication management would require standards. Additionally, those various frequencies have different capabilities and limits, such as reading .
The U.S. Food and Drug Administration today approved the first drug in the U.S. with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible.
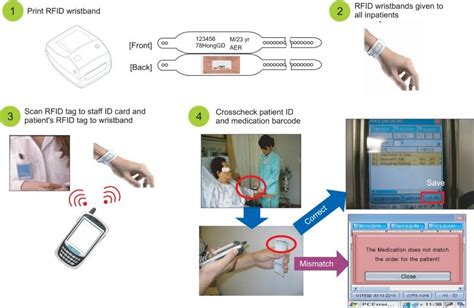
So, a “universal” RFID tag for medication management would require standards. Additionally, those various frequencies have different capabilities and limits, such as reading through glass, or water. There are basically two types of RFID tags, passive and active.use RFID to manage drug shortages or recalled medications (see Workflow section). RFID offers the possibility of seamlessly capturing data in the electronic health record (EHR) at the unit-of-use level and providing accurate inventory and patient records. Controlled substance (CS) inventory management is an area where RFID could provide
In 2004, the United States Food and Drug Administration approved a radiofrequency identification (RFID) device that is implanted under the skin of the upper arm of patients and that stores the patient's medical identifier. This paper mainly focuses on three types of intravenous mixtures: tocilizumab, abatacept, Remicade ® (infliximab) and Inflectra™ (biosimilar infliximab), dosified in the Pharmacy Service and administrated at the Day Hospital of A Coruña University Hospital (CHUAC), prescribed for rheumatological, neurological, or digestive diseases.
Thoughts on use of RFID in Medication Management
Radio frequency identification (RFID) has been considered one of the most promising technologies in healthcare and has been recognized as a smart tool with the potential to overcome many challenges that health care encounters such as inaccurate pharmaceutical stock, inability to track medical equipment, difficulty in tracking patient locations .

An RFID chip is typically a simple piece of hardware with a unique identifier and a small amount of read/write storage. Currently, this storage is insufficient for significant medical information, so the chip usually stores only a patient identifier, which links . This scoping review examines the state of RFID technology in the healthcare area for the period 2017-2022, specifically addressing RFID versatility and investigating how this technology can contribute to radically change the management of public health.
The RFID-based medication adherence intelligence system (RMAIS) is composed of an RFID reader, scale, microcontroller, liquid crystal display panel, and a motorized rotation platform [44,67]. The patient’s pill bottles are labeled with an RFID tag that stores the medication’s information, such as the medication name and appropriate dose [ 67 ].
1. Introduction. Nowadays, all of the activities involved in the care process of the patient are important. This translates into a chain of tasks in which each one is a vital link in the protection of the patient.
FDA approves pill with sensor that digitally tracks if patients
The U.S. Food and Drug Administration today approved the first drug in the U.S. with a digital ingestion tracking system. Abilify MyCite (aripiprazole tablets with sensor) has an ingestible.
So, a “universal” RFID tag for medication management would require standards. Additionally, those various frequencies have different capabilities and limits, such as reading through glass, or water. There are basically two types of RFID tags, passive and active.use RFID to manage drug shortages or recalled medications (see Workflow section). RFID offers the possibility of seamlessly capturing data in the electronic health record (EHR) at the unit-of-use level and providing accurate inventory and patient records. Controlled substance (CS) inventory management is an area where RFID could provide
In 2004, the United States Food and Drug Administration approved a radiofrequency identification (RFID) device that is implanted under the skin of the upper arm of patients and that stores the patient's medical identifier. This paper mainly focuses on three types of intravenous mixtures: tocilizumab, abatacept, Remicade ® (infliximab) and Inflectra™ (biosimilar infliximab), dosified in the Pharmacy Service and administrated at the Day Hospital of A Coruña University Hospital (CHUAC), prescribed for rheumatological, neurological, or digestive diseases.
Radio frequency identification (RFID) has been considered one of the most promising technologies in healthcare and has been recognized as a smart tool with the potential to overcome many challenges that health care encounters such as inaccurate pharmaceutical stock, inability to track medical equipment, difficulty in tracking patient locations .An RFID chip is typically a simple piece of hardware with a unique identifier and a small amount of read/write storage. Currently, this storage is insufficient for significant medical information, so the chip usually stores only a patient identifier, which links .
This scoping review examines the state of RFID technology in the healthcare area for the period 2017-2022, specifically addressing RFID versatility and investigating how this technology can contribute to radically change the management of public health.The RFID-based medication adherence intelligence system (RMAIS) is composed of an RFID reader, scale, microcontroller, liquid crystal display panel, and a motorized rotation platform [44,67]. The patient’s pill bottles are labeled with an RFID tag that stores the medication’s information, such as the medication name and appropriate dose [ 67 ].
rfid tag yeezy
rfid tag phone
Here’s how you can read NFC tags with your iPhone: Activate NFC Reader Mode: Ensure that your iPhone is in NFC reader mode, allowing it to detect and interact with nearby .
rfid chip in medication|Thoughts on use of RFID in Medication Management